Transmembrane (TM) proteins are essential conduits of extracellular information, regulating cell signalling. Their position at the boundary between the extracellular and intracellular environments also makes them prime targets in the early stages of host-pathogen interactions. Therefore, these membrane proteins play a key role in normal and pathological human physiology.
In this context, our research aims to characterize the molecular basis of ligand binding, activation mechanism and regulation of TM proteins using molecular pharmacology and an integrated structural biology approach combining biochemistry, NMR spectroscopy, crystallography, cryoEM, structural mass spectrometry (native and HDX) and bioinformatics. We are particularly interested in proteins with high potential for the development of therapeutic molecules, such as G protein-coupled receptors (GPCRs) and enzymes controlling ceramide homeostasis. Building on this knowledge, we use chemoinformatics approaches, and in particular AI, to identify original ligands that can modulate the activity and function of TM proteins. These ligands constitute both tools for studying the function of proteins of interest in vivo, and a starting point for the development of innovative drug discovery programs.
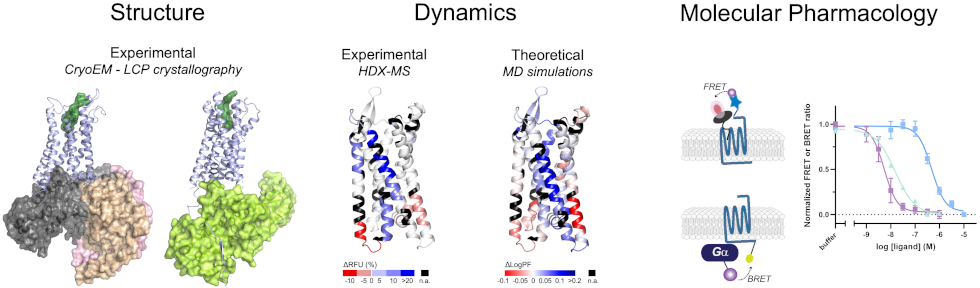
Structure, dynamics and molecular pharmacology of GPCR signaling complexes analyzed using a combination of Cryo electron microscopy and Lipidic cubic phase crystallography (CryoEM and LCP), Hydrogen-Deuterium exchange mass spectrometry (HDX-MS), molecular dynamics simulations (MD) and FRET- or BRET-based assays in living cells.
Integrated Structural Biology Approachs
Structural analyses of receptors and signaling complexes using biochemistry, fluorescence, NMR spectroscopy, X-ray crystallography, cryo-Electron Microscopy ans Native Mass Spectrometry.
